The Race for the Awaited Vaccine
This article reviews the global efforts aimed at creating a safe and reliable vaccine to control the outbreak of the emerging coronavirus, and discusses the economic, political and security dimensions surrounding the vaccine development process in light of the continued increase in the number of infections around the world and the overburdening of the health system and the economy due to the repercussions of the pandemic.
by STRATEGIECS Team
- Publisher – STRATEGIECS
- Release Date – Oct 7, 2020
This is the ninth article in the series of articles that are being published successively in the file "Files on the G7 Table"
The Covid-19 pandemic hangs heavy on most aspects of life for billions of people around the world. Governments and peoples are on the horns of a dilemma; to choose between openness and accepting the increasing number of cases, or closure and bearing high economic and social costs. Hence, it was imperative to seek a radical cure that would restore life to normal or at least ensure a balance between health considerations and economic imperatives.
Immunologists stress that unilateral measures taken by states are ineffective in overcoming outbreaks of infectious diseases unless efforts are coordinated regionally and internationally; for in a globalized international system, separate states cannot be closed and secluded behind their borders independently of the surrounding countries.
The disparity in the spread of the pandemic between neighboring countries has caused a major setback for some countries, such as Jordan, which after recording "zero local cases" for several rounds has seen reported cases explode into numbers many times higher than those recorded at the beginning of the first wave. The competent authorities attributed this to border crossings, as Jordan maintained the flow of exports and imports with surrounding countries, although with strict preventive measures; however, it did not succeed in preventing the virus from infiltrating, in what has become known as the "Jaber loophole."
Governments and competent medical committees are aware of the need to build "global" social immunity to break the grand chain of infections. Therefore, major pharmaceutical companies have been engaged in developing a vaccine amid many demands for fair allocation and the avoidance of any “monopoly” in favor of industrialized or rich countries that are able to afford it at the highest price.
The Director-General of the World Health Organization, Tedros Adhanom Ghebreyesus, in a press conference in August 2020, called nations of the world to refrain from what he called "vaccine nationalism." This is because an effective response requires global cooperation in a way that ensures the delivery of vaccines in the first instance to the most vulnerable groups, NOT their delivery to all populations in specific countries, as WHO stressed the need to give priority to health and social care workers.
To this end, the global COVAX initiative has been activated as a major database of potential vaccines. This initiative has become an "advanced operations room" to coordinate and organize related efforts, and most importantly, provide the necessary funds and expertise for this purpose. The initiative acts as a trusted link between vaccine developers and its member states the number of which reached 172 by August 2020, according to data from WHO, which is participating in managing "COVAX" with the Coalition for Epidemic Preparedness Innovations "CEPI" and the Global Alliance for Vaccines and Immunization "GAVI".
This mechanism has gained additional momentum after the commitment of governments wanting to obtain doses of the promised vaccine to pay a down payment before October 9, 2020. The initiative has set a main goal of providing two billion safe doses of the new vaccine before the end of 2021. The initiative promises to fairly distribute the vaccine in the first stage in proportion to the number of the population, provided that it expands its distribution - in later stages - according to the considerations of the epidemiological situation in the most affected countries.
As with most issues related to the novel coronavirus, ambiguity and anticipation continue to surround the actual date on which the promised vaccine will be distributed. If the developing companies succeed in the coming months, they will achieve a record in inventing a new vaccine, AS such an innovation requires approximately 3-5 years through which the vaccine passes gradual stages of clinical trials on humans. These trials are now being conducted by pharmaceutical companies, but in an intensive manner.
some specialists are afraid of a "leapfrog" in the race to innovate the vaccine. In May 2020, The New England Journal of Medicine published a scientific article entitled "Developing Covid-19 Vaccines at Pandemic Speed," in which it reviewed the main challenges facing the manufacturing of new vaccines; namely:
First, the optimal technique to generate immunity against the virus is a matter of medical debate. It is developed through stimulating the immune system upon injection to form an immune reaction similar to the real infection, as the vaccine is composed of either the protein chain of a weak virus, or the active part of the virus that attaches to and penetrates into the body's cells. However, this potential formulation is the subject of ongoing medical debate.
Second, it is possible for the immune reaction, when the vaccine is injected, to cause serious damage that exceeds that of the infection with the virus itself. As the acquired Acquired antibodies may cause complications in other parts of the body. Therefore, before any vaccine is approved by who, thousands of participants must be subjected to clinical trials.
Third, it is still too early to predict the ability of a particular vaccine to provide prolonged immunity. Also, it is not possible to say with certainty that the necessary immunity will be provided by a single dose of a specific formulation.
What may decrease the hope of securing an effective vaccine soon is the possibility of a mutation in the genetic sequence of the virus. Since any vaccine is designed to deal with a strain of known composition, if there is any qualitative change, the "outdated" vaccine will not work efficiently. In this regard, the medical director of the National Foundation for Infectious Diseases, Dr. William Schaffer, revealed to CNBC, in a report published in October 2015, that the influenza season in 2014 was abnormal, as a strain which caused nearly all cases of influenza was mutated; that is, it is different compared to the strain based on the composition of which the vaccine was developed.
Accordingly, the process of finding a solution appears complex and requires a delicate balance between accuracy and speed, the matter which the parties involved in the innovation of the vaccine emphasize, thus, it is by no means possible to risk safety standards and personal welfare of hundreds of millions who will be injected. This concern is evidenced by the pause of the AstraZeneca Oxford clinical trials after a participant had shown severe symptoms.
Flu-Conomics
In a report published in January 2013, Rueters news agency quoted facts and figures about the repercussions of epidemics on the global economy. For example, the relatively limited outbreak of the SARS coronavirus caused losses estimated at $47.8 billion in China, US and Europe, although the health crisis was not dangerous as the virus caused the death of only 916 people and its main wave lasted less than a year. It is not only emerging epidemics that cause losses, the influenza season causes the loss of 111 million working days in the US annually, according to estimates by the US Department of Health and Human Services.
A report issued by the World Bank in 2008 provided preliminary forecasts of the global GDP loss in the event of a large-scale pandemic outbreak. It expected that the global total value of GDP will decrease by 0.7% if the world is exposed to what is similar to the 1968-1969 Hong Kong flu, and it will decrease by 2% if the outbreak is similar to the Asian flu of 1957. What should have been considered an early warning alarm is the report's projection of losing 4.8 % of the global economy with losses exceeding 3$ trillion and an unprecedented global recession in case of an outbreak similar to the Spanish flu 1918-1919.
These numbers show that most forecasts confirm the magnitude of the economic damage of any pandemic outbreak. However, what was needed to limit the damage had been the actual preparation for such a scenario which was always excluded from the list of the most prominent global threats. This is due to what psychologists describe as a kind of "normalcy bias", in which individuals as well as groups, such as governments, refrain from dealing seriously with the possibility of a sudden change; since nothing in everyday life foretells such an imminent change.
Perhaps the flu-conomics did not receive serious attention before the Covid-19 pandemic, because humanity had not experienced any global pandemic since the outbreak of the Spanish flu. Before that, developed vaccines have quickly led to the containment and encirclement of any outbreak of epidemic foci around the world.
Certainly, these vaccines are considered to be a key pillar of the epidemic’s reaction; not only because they speed up the return to normal life, but because they also draw a map of major drug centers around the world that run a pharmaceutical market whose value was estimated in 2018 at $ 952.5 billion, according to Global Data for data analysis and advisory.
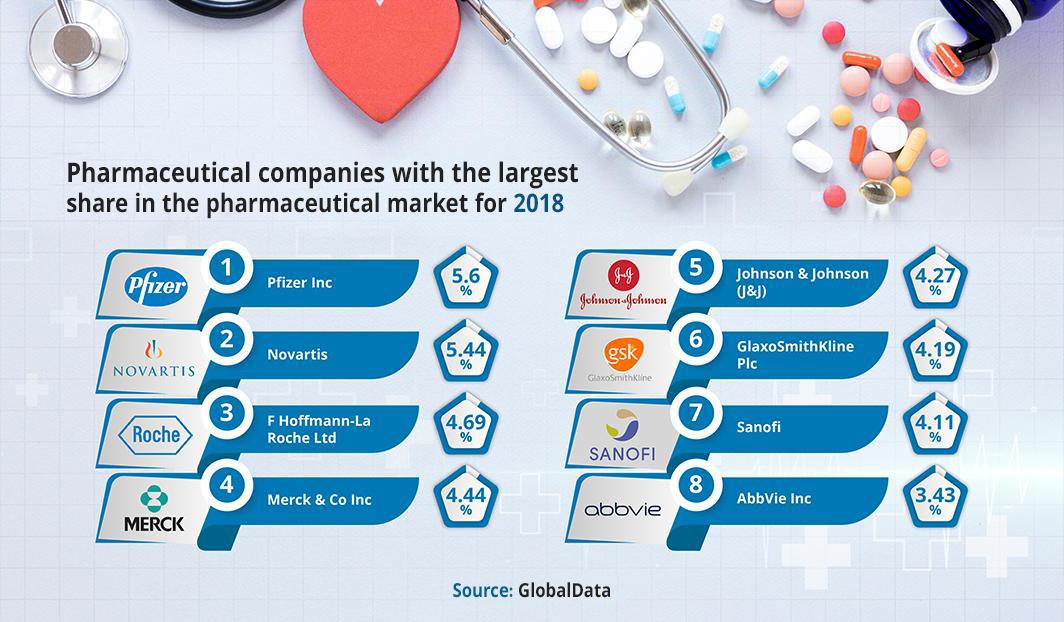
It is expected that sales of the awaited vaccine will generate huge profits for the developing companies estimated at $ 30 billion after tax, according to Fierce Pharma, a pharmaceutical industry reporting association. In its report, published in August 2020, the association expected that 40% of the market’s needs would be met by Moderna, a leading company in the development of cures using biotechnology , and that Novavax, a company also specialized in the field of biotechnology, would provide the market with 20% of its needs. The rest of the companies that will succeed in developing a vaccine, such as Pfizer, Johnson & Johnson, and AstraZeneca, will get the remaining 40%.
If the pharmaceutical companies achieve financial results close to expectations, then they will have achieved record profits compared to those of previous vaccines. For example, sales of the H1N1 vaccine in 2009 amounted to nearly $ 3.3 billion, according to data from Kolorama Information, a healthcare market research company.
The profits of the H1N1 vaccine were contrary to expectations, especially since its development costs were high, not to mention the manufacturing and logistical costs and the consequent reluctance of investors to engage in the vaccine industry because the failure rates of experiments are high and the investment return of vaccine innovation projects cannot be certain.
In addition, the spending - whether governmental or personal - on purchasing the vaccine was at the expense of purchasing other vaccines and medicines, such as those of seasonal flu. That being the case, sales of swine flu vaccine did not constitute a qualitative addition to the overall prevailing drug sales, but rather took a significant proportion of public spending - already intended - on medicines.
Consequently the novel coronavirus vaccine may not cause a boom in the profits of the pharmaceutical sector as a whole, but it may lead to the concentration of profits in the hands of a specific group. This is not surprising, as the world with all its entities is searching for a radical cure for this novel virus, which means that a multidimensional competition is blazing to obtain the advantages of the "vaccine in development".
Positive cooperation or negative competition?!
Without providing conclusive scientific evidence, and amid western center’s suspicion, Russian President, Vladimir Putin, announced, in August 2020, the registration of the first vaccine against the novel coronavirus, called "Sputnik V" after the name given to the first Russian satellite that was sent into space in 1957, making reference to winning the race for vaccine in a similar manner to the space race that Russia had won.
After examining the progress and approval of the Russian vaccine, it became clear that it has passed the second stage and, according to Russian instructions and regulations, it is not permissible to initiate the third stage in which the vaccine is tested on tens of thousands of volunteers without being officially approved. In other words, the confusion raised may be due to a "theoretical" and formal differences regarding the stages of vaccine adoption; as WHO and most countries of the world do not approve a vaccine until after the end of the third phase of trials.
The “propaganda and counter-propaganda” that accompanied the adoption of the Russian declaration led to the clear entry of Covid-19 vaccines onto a political stage that leads to the belief that the vaccine will be an issue of a political concern as much as it is a medical and economic one. In this regard, BBC, in a report published in August 2020, quoted the professor of global health law at Georgetown University in the US, Lawrence Gostin, "I have never seen the political stakes for a medical product being so intense. The reason the Covid-19 vaccine has taken on such political symbolism is that the superpowers have seen the vaccine as projecting their scientific prowess, actually validating their political system as superior."
It is not only international considerations that are driving the politicization of the awaited vaccine, but also the local turmoil caused by the pandemic, especially in regard to economics. The United States is the most prominent example of this situation, as it is still on the list of countries with the most recorded infections and its economy has confronted a violent shock that almost destroyed the economic gains achieved during the era of US President, Donald Trump. This accordingly led some to count the novel coronavirus as responsible for Trump's loss in the elections if the Democratic candidate, Joe Biden, wins.
Prior to the presidential elections scheduled for November 3, 2020, the US center for Disease Control and Prevention (CDC) called on the authorities of the states to prepare logistically and arrange the vaccine distribution centers that may be granted a preliminary permit to be used in emergency situations before it is officially approved and broadly distributed.
This political polarization has cast a shadow over the protocol for the vaccine approval in the United States, as the medical community is afraid of exerting pressure to interfere with the production date of the vaccine, whether by speeding it up or even pressing to delay it until after the American elections, so that the “vaccine" does not affect the voting decisions of the American voters.
Trump did not hesitate to criticize the FDA and accuse it of seeking to delay the results of vaccine tests until after November 3, describing it in a tweet he posted in August 2020 as a deliberate intervention by the "deep state" against his administration. In other words, the promised vaccine is not immune to the traditional debate that has recently dominated US policy between the White House and the "deep state". However, the American medical establishment always stresses its dissociation from political disputes and that the approval of the vaccine will be "a decision related to science, medicine and data," as the head of the FDA, Stephen Hahn, said in an interview with Financial Times in August. 2020.
In addition to the political dimensions, the vaccine development process has acquired a security dimension related to cyber security. It was announced that several espionage operations have been monitored against Western parties working to develop Covid-19 vaccines. The New York Times described what is taking place in the cyberspace as a "fierce war" that recalls the race for outer space between United States and the Soviet Union. The newspaper added that "hackers belonging to Chinese intelligence services" targeted what are supposed to be easy targets for obtaining vaccine data, like universities, as penetration of such entities is easier than penetrating the companies developing the vaccine.
Away from economic competition, political turmoil and security privacy, the G7 must unite in a deeper way to stand up to the pandemic and coordinate efforts towards reaching a cure that all humanity awaits and expand the investment in the "Access to COVID-19 Tools " initiative (ACT). So far, only $ 3 billion has been invested out of the $ 35 billion needed to have such a large-scale impact.
Even with regard to the aforementioned COVAX initiative, some countries, such as the US, have reservations about joining it because the main criterion on which the initiative is based, which is to ensure vaccines for “some people in all countries and not all people in some countries,” conflicts with the goal of vaccinating American residents first.

STRATEGIECS Team
Policy Analysis Team
 العربية
العربية